Early treatment can prevent vision loss
The MUSC Storm Eye Institute was a participant in a clinical trial that has provided doctors with improved prognostic indicators and treatment options for retinopathy of prematurity (ROP), a blinding disease that affects premature, low birthweight infants. ROP spurs the growth of abnormal blood vessels in the back of the eye. These vessels leak fluid and blood and scar the nerve tissue inside the eye, increasing the risk of retinal detachment and severe vision loss in infants.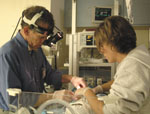 Dr.
Richard Saunders, pediatric ophthalmologist, and Lisa Langdale, R.N., ROP
nurse coordinator, examine a premature infant using indirect ophthalmoscopy
to screen for Retinopathy of Prematurity (ROP).
Dr.
Richard Saunders, pediatric ophthalmologist, and Lisa Langdale, R.N., ROP
nurse coordinator, examine a premature infant using indirect ophthalmoscopy
to screen for Retinopathy of Prematurity (ROP).
Because it follows an unpredictable course, ROP presents doctors with difficult treatment decisions. In many infants the disease spontaneously regresses and spares vision. However, in some infants ROP progresses, resulting in serious visual impairment. Although current therapy, commonly performed using a diode laser, usually halts its progression, many infants are still blinded by the disease.
Due to a lack of clinical criteria to predict which patients will ultimately develop severe vision loss from ROP, ophthalmologists were forced previously to defer treatment until it was clearly indicated. Unfortunately, delaying therapy can leave infants who might benefit more from early treatment with poor visual outcomes.
“I see blindness as the major threat of severe prematurity,” said Richard Saunders, M.D., pediatric ophthalmologist and principal investigator for the study at the MUSC site. “ROP is a leading cause of vision loss in children. Some premature infants will grow up with multiple handicaps, and if you throw blindness into the mix, you have a child facing major obstacles.”
Other MUSC participants in the study were Dilip Purohit, M.D., director of neonatology, Millicent Peterseim, M.D., pediatric ophthalmologist, and Lisa Langdale, R.N., the MUSC study center coordinator and a neonatal ICU nurse.
The Early Treatment for Retinopathy of Prematurity (ETROP) study was sponsored by the National Eye Institute (NEI), a part of the National Institutes of Health (NIH). Results of the study, published in the December issue of the Archives of Ophthalmology, demonstrated that premature infants, who are at the highest risk for developing vision loss from ROP, will retain better vision when therapy is administered in the earlier stage of the disease. This treatment approach was found to be better than waiting until ROP has reached the traditional treatment point, called “threshold.” Just as importantly, the study also established the value of an improved risk assessment model to identify more accurately those infants who are at the highest risk for developing severe vision loss from ROP.
“Premature, low birthweight infants face a host of medical complications with lifelong consequences. The results of this study allow us to improve treatment for ROP and, hopefully, the quality of life for children who most need sight-saving therapy,” said Paul A. Sieving, M.D., Ph.D., director of the NEI.
Each year ROP affects an estimated 14,000-16,000 premature, low-birthweight infants in the United States and thousands more worldwide. Of these cases, approximately 1,500 infants in the United States will develop severe ROP that requires treatment. Despite available treatment, about 400-600 infants with ROP still become legally blind each year. Researchers have identified birthweight of 2.75 pounds (1250 grams) or less as a major risk factor for developing ROP.
The previous standard treatment threshold for ROP hinged on the disease having progressed enough that the risk of developing retinal detachment approached 50 percent. This determination is made based of specific findings on ophthalmic examination.
As part of the ETROP study, a new computerized risk model, developed by NEI-supported researchers, was used to identify high-risk infants early in the disease. The risk model assessed birthweight, ethnicity, being a single or multiple birth baby, gestational age, ophthalmic exam findings, and whether the infant had been born in a hospital that participated in the study.
“This new risk assessment model helps detect infants who have a higher risk of blindness, justifying moving the treatment window forward a week or two and giving physicians a defensible reason for early treatment,” said Saunders, who also serves on a subcommittee for practice guidelines for screening for retinopathy of prematurity of the American Academy of Pediatrics. “The study results also indicate that in many cases watchful waiting without immediate treatment of developing disease is the preferred approach. Laser therapy involves destruction of the peripheral retina, which is not benign. So it is important not to treat a large number of infants that are likely to do well anyway. Clinical judgment and experience of the physician are critical in sorting out the many factors involved in a decision to treat infants early.”
Once identified, the infants were then assigned randomly either to treatment at the standard threshold or to early treatment. Researchers found that early treatment significantly reduced the likelihood of poor vision from 19.5 to 14.5 percent at about one year of age. Early treatment also considerably reduced the likelihood of structural damage to the eye from 15.6 to 9.1 percent.
Current treatments for ROP involve laser therapy or cryotherapy. Laser therapy uses heat from light energy while cryotherapy uses freezing temperatures to retard blood vessel growth. A consequence of these treatments, known clinically as retinal ablation, is a partial loss of peripheral or side vision. Nonetheless, treatment is valuable in preserving the most important part of sight—the sharp, central vision needed to read, see faces or perform detailed tasks that require hand-eye coordination.
The study will continue to follow these infants until age six to ensure that the benefits of early treatment persist through childhood.
The study was conducted at 26 centers in the U.S.
Catalyst Online is published weekly, updated as
needed and improved from time to time by the MUSC Office of Public Relations
for the faculty, employees and students of the Medical University of South
Carolina. Catalyst Online editor, Kim Draughn, can be reached at 792-4107
or by email, catalyst@musc.edu. Editorial copy can be submitted to Catalyst
Online and to The Catalyst in print by fax, 792-6723, or by email to petersnd@musc.edu
or catalyst@musc.edu. To place an ad in The Catalyst hardcopy, call Community
Press at 849-1778.