|
by Mary Helen Yarborough
Public Relations
A center responsible for bringing in millions of research dollars to
MUSC has a new name that reflects the institution’s lab-to-bedside
commitment in response to new federal funding initiatives.
On Dec. 19, the General Clinical Research Center (GCRC) became known as the Clinical and Translational Research Center (CTRC).
CTRC works in conjunction with the Support Center for Clinical and
Translational Science (Success) Center, which provides regulatory,
navigation, and participant recruitment support. Both centers operate
as components of the S.C. Clinical and Translational Research Institute
(SCTR).
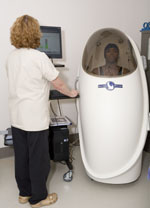 Research Support Center's Betsy Anderson, left, and Simone Chinnis demonstrate how the BodPod works. Research Support Center's Betsy Anderson, left, and Simone Chinnis demonstrate how the BodPod works.
“We
are pleased to have the GCRC become the Clinical and Translational
Research Center (CTRC) as part of the larger South Carolina Clinical
and Translational Research Institute (SCTR). The same excellent
clinical research support services will be offered, but services will
be expanded and better coordinated with other research support services
campus wide,” said Kathleen Brady, M.D., Ph.D., director of SCTR
Institute. Through this new relationship, an investigator can access a
much broader range of services, laboratory assays and outreach
activities through a centralized system that includes the CTRC, she
added.
Located in Suite 215 of the Clinical Sciences Building, the 9,200
square foot center has eight exam rooms, three procedure rooms, a
dental suite, and a DEXA bone density scanning suite. It offers
equipment, supplies and nursing/laboratory services needed for a wide
variety of clinical trials.
“We have recently acquired several new pieces of equipment that we hope
will be useful to investigators,” said Susan Sonne, PharmD, CTRC
director of operations. “Specifically, in addition to our DEXA machine,
we now have both a PeaPod and a BodPod, which evaluate body composition
and basal energy expenditure for infants (PeaPod) through adults
(BodPod). We will be expanding other services through the CTRC as well.
Investigators who are interested in possibly using the CTRC are invited
to attend the investigator drop in from 2 to 3:30 p.m. Dec. 19.” (See
announcement on page 2.)
All of the new equipment was purchased several months ago, prior to the recent state budget cuts.
“In these difficult economic times of state and NIH funding cuts, the
SCTR Institute is working hard to fill gaps and support researchers at
MUSC by continuing to provide and expand research support services.
Also the Success Center is available to help any researcher on campus
with hands-on guidance, training and support related to the research
process, regulatory approvals and recruitment,” said Royce R. Sampson,
CTRC finance and administration director.
“The CTRC will function like the GCRC did, awarding support (in the
form of nursing, laboratory, or nutrition support) for
investigator-initiated studies. The amount of support provided is
calculated on a per patient per visit basis. The current amount of
support is approximately $35-$40 per patient per visit,” said Lane
Glenn, the center’s resource coordinator.
“For example, if an investigator plans to enroll 100 patients and each
patient is expected to make one visit to the CTRC, the CTRC would
typically provide support that would add up to $3,500-$4,000 for the
study. However, all support requests must be reviewed and approved by
CTRC advisory committee.”
The CTRC also supports industry/corporate sponsored studies on a
fee-for-service basis, making it essentially self-supporting. Services
are charged on a per patient and/or per use basis, and include:
--Vitals/height/weight: $11
--EKG: $50
--Phlebotomy: $12.50
--Urine specimen: $10
--Urine drug screen: $20
--Boxed meal: $5
--Centrifuge: 70 cents/tube
--Specimen handling: 50 cents/tube
--Label/transfer/aliquot: $1/tube
--Sample shipping and packaging (frozen): $22/package
--DNA isolation (10ml): $13.50
--DNA sequencing: $10/sample
--DEXA scans (total body): $175/scan
--Informatics support: $50/hour
--Protocol start-up fee: $500
--Protocol review fee: $500
The cost of all of these services is usually determined on a study-by-study basis.
In addition to laboratory and nursing staffing, this 20-member team
also handles all ordering and maintaining the center’s entire supply
inventory, billing and payroll, database management, all while
assisting investigators with applying for CTRC support. All scheduling
is electronic. Investigators and/or coordinators can access CTRC’s
current Web site with their network identification and password. Once
in the site, patient registration links can be found.
After the investigator completes and submits all required forms, CTRC’s
registration desk receives the scheduling request electronically.
“To make things easier on everyone, researchers can call the Success
Center and the staff will link them into whatever SCTR services they
may need including CTRC,” said Ashlee Watts, SCTR marketing and
recruitment coordinator.
For more information on CTRC contact Glenn at 792-3357 or e-mail
glennj@musc.edu; or the Success Center, 792-8300, success@musc.edu.
Sonne can be reached at 792-5221; sonnesc@musc.edu.
Friday, Dec. 19, 2008
|



