|
|

|
New MUSC cellular therapy center expands, improves transplants
|
A
new and unique facility at MUSC uses a patient’s own cells to perform
life-saving procedures while erasing the problem of rejection and
reducing the need for full organ transplants.
The Center for Cellular Therapy (CCT) is the first center in South
Carolina to perform an autologous islet cell transplant for chronic
pancreatitis. The first patient, a woman from Aiken, underwent a CCT
procedure on March 9 that enabled insulin-producing islet cells
to be removed from her diseased pancreas and transplanted into her
liver.
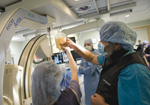 Dr.
Katherine Morgan and visiting consulting islet scientist Dr. Horacio
Rilo of Tucson, Ariz., check on the infused islet cells harvested from
a patient suffering from chronic pancreatitis. Interventional
radiologist Dr. Renan P. Uflacker prepares to reintroduce the patient’s
own insulin-producing cells into her liver using a catheter and
flouroscopy via the portal vein. The March 9 breakthrough procedure,
known as an autologous islet cell transplant, was performed to help a
48-year-old patient from Aiken and others diagnosed with chronic
pancreatitis. Dr.
Katherine Morgan and visiting consulting islet scientist Dr. Horacio
Rilo of Tucson, Ariz., check on the infused islet cells harvested from
a patient suffering from chronic pancreatitis. Interventional
radiologist Dr. Renan P. Uflacker prepares to reintroduce the patient’s
own insulin-producing cells into her liver using a catheter and
flouroscopy via the portal vein. The March 9 breakthrough procedure,
known as an autologous islet cell transplant, was performed to help a
48-year-old patient from Aiken and others diagnosed with chronic
pancreatitis.
This breakthrough procedure is the first in a series of innovative
therapies from the new CCT, which is a collaboration between MUSC,
Hollings Cancer Center (HCC), Department of Surgery, General Clinical
Research Center, Digestive Disease Center (DDC), Interventional
Radiology and Department of Pediatrics.
“The important work going on in this new center will include advanced
cellular transplants for patients with chronic pancreatitis and
pancreatic disease as well as cancer vaccine clinical trials,” said
Andrew S. Kraft, M.D., HCC director. “One of the most difficult steps
in translational cancer research is moving a therapeutic strategy from
the lab into human clinical use in a structured and safe manner. Having
this facility will greatly speed up the testing of state-of-the-art
cancer treatments.”
One of the reasons that HCC was able to attain the recent prestigious
National Cancer Institute cancer center designation is through the
development and support of innovative facilities such as the CCT.
CCT features a sterile facility called a clean lab in which researchers
produce clinical-grade cellular and tissue products for patient
therapies. This allows MUSC researchers to process anything from stem
cells to whole organs with the goal of fighting diseases such as
cancer, pancreatic disease and diabetes.
Cellular transplantation, used at only a handful of specialized centers in the country, is used to treat chronic pancreatitis.
Patients with chronic pancreatitis suffer from numerous medical
complications and severe pain. When medication is no longer effective,
patients often require surgery to remove part of the entire pancreas.
Removal of the pancreas also removes the ability to produce insulin,
leaving the patient with no glucose control and, ultimately, severe
type 1 diabetes.
In its maiden procedure, the MUSC CCT team achieved a breakthrough with
its first autologous transplant of islet cells. In this procedure, the
inflamed pancreas was removed in the operating room and taken to the
“clean” laboratory where specially-trained technicians used a
microscope and extracted the patient's insulin-producing islet
cells. Then an interventional radiologist and his team used fluoroscopy
to guide a catheter through the patient’s upper abdomen and into the
main blood vessel in the liver. The islet cells were infused back into
the liver where they are expected to lodge in blood vessels and begin
functioning like a miniaturized pancreas, producing and releasing
insulin. By infusing the patient's islet cells into the liver, they
will start producing insulin for the body and eliminate the onset of
diabetes.
“Being able to work in specialized teams in a coordinated effort was
very important to the success of this procedure,” said Renan P.
Uflacker, M.D., professor of radiology and director of
Vascular-Interventional Radiology. “It gives hope to this patient and
many other individuals diagnosed with chronic pancreatitis and other
diseases requiring specialized tissue and cell therapies. It is the
first step in a long-lasting, collaborative effort to provide novel
therapies for cancer patients and other important diseases such as
diabetes.”
Ordinarily, a severely or chronically inflamed pancreas is removed by a
surgical team. With islet cell autotransplantation, surgeons remove the
whole pancreas and use the patient’s own insulin-producing islet cells
by extracting and purifying them for injection later into the patient’s
liver where they create a new blood supply and perform their normal
function. Most patients do not need immunosuppressant drugs, because
they essentially receive their own tissue.
“The work and expertise of many individuals have come together in the
development of this program,” said Katherine Morgan, M.D., assistant
professor of surgery, DDC, who was part of the procedure team. “Each
part was absolutely essential, and I am amazed at the enthusiasm and
diligence shown from all parties including physicians (surgeons,
anesthesiologists, endocrinologists, radiologists), islet scientists,
nursing staff, physician assistants, residents and administrative
staff.”
Cancer vaccine clinical trials
Historically, cancer treatment primarily has relied on surgery,
radiation and chemotherapy. However, in the last decade scientists have
realized that cancer occurs because a patient’s immune system fails to
recognize cancer cells as foreign and does not attack them as it would
bacteria, for example.
“Using the body’s ability to defend against disease and heal itself is
an exciting frontier in treatment and research for many diseases,” said
Michael Nishimura, Ph.D., professor of surgery, microbiology and
immunology, CCT scientific director and HCC Immunology Program leader.
“In this lab we are, in essence, equipping a patient’s immune cells to
recognize and destroy cancer cells. We’ll be developing vaccines and
other kinds of cellular therapies to use now against various kinds of
cancer.”
MUSC
Center for Cellular Therapy scientific director Dr. Michael Nishimura
quickly transports the patient’s pancreas after it was removed to the
center’s clean lab for islet cell harvesting. The Center is a sterile
facility where islet researchers extract the islet cells from the
pancreas and later, place them back in the patient’s body.
Nishimura said the CCT already is running two FDA-approved trials—one
designed to prevent type 1 diabetes in patients who had a
pancreatectomy; and another designed to develop a therapeutic cancer
vaccine for patients with metastatic colon cancer. There are other
trials on the horizon, including breast cancer and pancreas cancer
vaccines as well as T-cell therapies for patients with melanoma and
liver cancer.
More than $3.5 million dollars was provided to create the CCT by HCC,
MUHA, GCRC and Department of Surgery. Additionally, the clean cell
facility from which the CCT was created initially was made possible
with a $1 million gift from the Abney Foundation to treat juvenile
diabetes via allogenic islet cell transplants.
Friday, March 27, 2009
|
|
|




