|
|
|

|
Prenatal studies make history
|
by Dawn Brazell
Public Relations
Becoming part of obstetrical history with its participation in a second
national fetal growth study, MUSC will help establish the gold standard
for what is normal growth and development of twins.
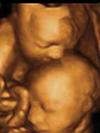 MUSC initiates its
second landmark obstetrical ultrasound study - this
time focusing on twins. The photo above is a 4D ultrasound image of
Jennifer Montgomery’s twin boys at 25 weeks of gestation. MUSC initiates its
second landmark obstetrical ultrasound study - this
time focusing on twins. The photo above is a 4D ultrasound image of
Jennifer Montgomery’s twin boys at 25 weeks of gestation.
MUSC is one of five hospitals chosen to be a part of the National Twins
Study, which will begin recruiting women this month to be a part of
this research sponsored by the Eunice Kennedy Shriver National
Institutes of Child Health and Human Development.
Roger Newman, M.D., the study’s principal investigator, said this is
the second nationally important study using obstetrical ultrasound that
has been undertaken by the Maternal-Fetal Medicine service at 135
Cannon Place, a part of MUSC’s Department of Obstetrics and Gynecology.
“It’s important for us to be part of these studies. The selection of
the study sites was to create geographic and ethnic diversity in terms
of recruitment. What also makes us a good selection is the quality of
the prenatal ultrasound services that we offer. Our research
infrastructure in the Department of OB-GYN is very strong and we are
able to deliver on what we say we can do.”
 The
hospital began recruiting to the National Standard for Normal Fetal
Growth Study, also sponsored by the National Institutes of Health, in
2009. That study runs through March 2012 and uses ultrasound to
redefine what represents normal fetal growth in healthy women pregnant
with one child. The
hospital began recruiting to the National Standard for Normal Fetal
Growth Study, also sponsored by the National Institutes of Health, in
2009. That study runs through March 2012 and uses ultrasound to
redefine what represents normal fetal growth in healthy women pregnant
with one child.
Dr. Roger Newman
displays the T-shirt that is given to the study’s participants.
Newman said both of these studies are important because they rely on
the latest technologies in ultrasound imaging, an important part in
assuring normal fetal growth, which is critical to the long-term health
and well-being of the child.
The studies will help set a comparative gold standard to determine if
fetal growth is normal, restricted or excessive. Most current growth
standards are based on fairly small populations, and many of these
studies were performed 30 to 40 years ago with ultrasound technologies
inferior to what is available today. Another complicating factor is
that those studies included women who had risk factors for impaired
fetal growth. A true gold standard should be based upon fetal growth in
low risk women without medical or obstetrical complications, he said.
The National Twins Study will be recruiting 500 patients from five
centers nationwide, 50 from MUSC, while the fetal growth study will be
recruiting 2,400 women, about 500 from MUSC. Investigators are
collecting data that will allow them to develop customized fetal growth
curves that can be adjusted for maternal factors such as age, race,
parity, weight and height as well as for fetal gender. These studies
will set new standards for what represents normal fetal growth based on
individual maternal and fetal variables using ultrasounds instead of
the previous standard, which involved measuring the fundal height, he
said. The lack of quality, prospective longitudinal studies has left
major gaps in the understanding of what should be considered normal
versus abnormal growth.
“Each baby will be evaluated against its own individual growth
potential—not against some standard set in some other population. When
you’re dealing with millions of pregnancies, even small differences
become magnified within an entire population. Your baby should not be
evaluated using the same growth curve as another woman who is a
different size or ethnicity.”
Newman said the new standards will help doctors know better when they
should or should not intervene in a pregnancy. The twins study also
will answer important questions about differences between single and
twin gestations, between identical and fraternal twins and what degree
of growth discordance between co-twins is clinically acceptable.
“It’s a reflection of the high quality of prenatal ultrasound offered
at MUSC that our Prenatal Wellness Center was chosen for these
NIH-funded, national studies,” said Newman. “We’ve developed a
nationally- recognized prenatal diagnostic center, which enjoys a great
reputation. We see referrals from the lower half of South Carolina.”
In addition to MUSC, these two studies also are being performed at
Columbia University in New York City, Northwestern University in
Chicago, the University of California at Irvine, and the Women &
Infants Hospital in Providence, R.I. All of the participating centers
underwent rigorous pre-selection review and credentialing of both the
sonographers and investigators. There also is an ongoing quality review
of submitted scans.
 Newman
likes it that South Carolina women and their babies will be helping to
set the new standards. He holds up a T-shirt with the study’s slogan—
‘This is one of the 3,000 most important babies in the U.S.’ Newman
likes it that South Carolina women and their babies will be helping to
set the new standards. He holds up a T-shirt with the study’s slogan—
‘This is one of the 3,000 most important babies in the U.S.’
“It’s a great credit to our center and our university to be a part of
this. We’re excited to be participating in this.”
Registered nurse
Carolyn G. Williams holds up the onesie that babies in
the prenatal studies get to wear.
MUSC prenatal study requirements
Want to be a
study participant?
National Standard for Fetal Growth Study Pregnant women eligible for
the study must be:
- in the first trimester (less than 14 weeks) q
have a known last menstrual period
- be a nonsmoker
- and be free of any other significant medical or
obstetrical complications.
National Twins Study
Requirements for the National Twins Study are not quite as rigorous.
Smoking and other high-risk conditions are not exclusions. To be
eligible, mothers of twins need to be:
- in the first trimester (less than 14 weeks)
- and have a known last menstrual period.
Women from minority
groups especially are needed. One of the goals of these studies is to
recruit equal numbers of Caucasians, African- Americans, Hispanics and
Asians so that the results accurately reflect the diversity of the
national population.
What to expect?
Once enrolled, women receive:
- A total of six ultrasound exams during their
pregnancy, which include a comprehensive evaluation with 2-D and 3-D
images of all fetal and maternal structures.
- At each ultrasound visit, mothers will be
interviewed, measured (including the fundal height), complete a 24-hour
dietary recall and, on some visits, asked to provide a small blood
sample.
- After delivery, both and mother and baby will
be physically measured as part of the newborn assessment.
There are no costs associated with participation in these studies as
funding for all study-related procedures and ultrasounds is being
provided by the National Institutes of Health.
Compensation is offered for the time commitment involved. Each
participant also will receive a CD, which includes all of the 2-D and
3-D images obtained during ultrasounds. Participants will receive a
T-shirt with the National Fetal Growth Study logo recognizing their
participation. The mother’s T-shirt states: ‘My baby is one of the
3,000 most important babies in the United States’ and the baby’s onesie
states: ‘I am the standard by which all other babies are judged.’
For more information about the National Fetal Growth Study or the
National Twins Study, call any of the MUSC prenatal care sites and ask
to speak to a research coordinator.
- Carolyn Williams (792-0349) is available for
women receiving prenatal care at Cannon Place, Northwoods or at MUSC
Family Medicine.
- Holly Boggan (876-1434) sees patients at the
East Cooper Women’s Center practice.
- Sarah Cordell (792-6654) sees women at Cannon
Place downtown as well as the West Ashley Women’s Health office.
Friday, Jan. 21,
2011
|
|
|




 The
hospital began recruiting to the National Standard for Normal Fetal
Growth Study, also sponsored by the National Institutes of Health, in
2009. That study runs through March 2012 and uses ultrasound to
redefine what represents normal fetal growth in healthy women pregnant
with one child.
The
hospital began recruiting to the National Standard for Normal Fetal
Growth Study, also sponsored by the National Institutes of Health, in
2009. That study runs through March 2012 and uses ultrasound to
redefine what represents normal fetal growth in healthy women pregnant
with one child. Newman
likes it that South Carolina women and their babies will be helping to
set the new standards. He holds up a T-shirt with the study’s slogan—
‘This is one of the 3,000 most important babies in the U.S.’
Newman
likes it that South Carolina women and their babies will be helping to
set the new standards. He holds up a T-shirt with the study’s slogan—
‘This is one of the 3,000 most important babies in the U.S.’