|
In what could be a
science fiction scenario, Dvora Beeri became MUSC's first patient to
undergo brain surgery March 29 as part of a clinical trial to see the
effectiveness of CERE-110, a new type of gene therapy treatment for
patients with Alzheimer's disease.
By Dawn Brazell
Public Relatins
Jacobo E. Mintzer M.D.,
co-principal investigator with neurosurgeon Istvan Takacs, M.D., said
Beeri is the first in the state and the 19th in the world to have the
procedure done as part of a Phase 2, double-blind study involving
patients with mild to moderate Alzheimer's disease.
"We're skeptically
optimistic. We're opening a new door that we don't know where it's
going to lead us, but we're opening it locally in a way that puts us on
par with the top centers in the world," Mintzer said.
 Dr. Jacobo
Mintzer Dr. Jacobo
Mintzer
MUSC is one of 10 leading
centers across the U.S. that has been selected to be a part of the
study, which is being conducted in collaboration with the Alzheimer's
Disease Cooperative Study (ADCS). Mintzer, who is director of the
Department of Neurosciences a Division of Translational Research, said
the trial is a sign of the success of ADCS, a national research
consortium funded by the National Institute on Aging that conducts
multi-center clinical trials.
Given the prevalence and
devastation of Alzheimer's disease, this work required a network of
sites with state-of-the-art capabilities where any new treatment or
approach could quickly and effectively be put in the pipeline and
tested, Mintzer said. It was noticed that true innovation wasn't
necessarily coming from pharmaceutical companies but from small biotech
companies who oftentimes did not have the resources to move the science
forward.
The ADCS selected the 30
top centers in the country to form a network to aid these small
companies and implement clinical trials on a national level to move
this science forward faster through its grant system. Of those centers,
10 were selected for this study.
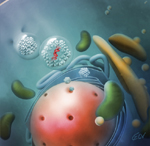 Gene therapy involves nerve growth
factor being injected into a critical area of the brain called the
Nucleus Basalis of Meynert. Medical
illustration by Emma Vought Gene therapy involves nerve growth
factor being injected into a critical area of the brain called the
Nucleus Basalis of Meynert. Medical
illustration by Emma Vought
The science being promoted
in this case studies how best to get NGF or nerve growth factor to a
critical area of the brain called the Nucleus Basalis of Meynert (NBM),
a brain region where cell degeneration occurs in Alzheimer's disease.
CERE-110 is composed of an adeno-associated viral vector carrying the
gene for NGF, a naturally occurring protein that maintains the survival
of nerve cells in the brain.
CERE-110 is surgically
injected into the NBM, where it is hoped the delivery of NGF gene using
a "virus" will allow NGF to replicate itself through the patient's own
cell machinery and stop the progression of the disease or even
potentially cause improvements.
"We have to get it in the
right place in a way the cell can incorporate it," said Mintzer. "We
need to get the cell to generate it by itself on an ongoing process.
The only way you can do that is by injecting new DNA into a cell."
 Beth Safrit, left, and Dvora Beeri Beth Safrit, left, and Dvora Beeri
Beth Safrit, nurse
practitioner III and clinical director of the Department of
Neurosciences Alzheimer's Research and Clinical Programs, will oversee
the monitoring of the five to 10 patients who will be participating in
the trial. The patients are followed for 24 months.
"It's the only thing out
there that can offer any true hope of improving," she said of the
study, which accepts patients who have only a mild level of
Alzheimer's. "If these patients can stabilize themselves or maybe even
get a little bit better with this one-time treatment, because that
little virus is producing for the rest of their lives, it's an amazing
thing. If it proves to be effective, it's going to change their lives
forever."
Mintzer said one of the
main challenges is pushing the science forward, while managing people's
expectations. It will be years before a therapy can be developed should
the therapy prove effective. Having begun his research into Alzheimer's
disease in 1981, Mintzer said he's seen impressive changes from when it
was difficult just trying to convince people that such a disease
existed.
"Now we have come to a
moment where we are inserting new genetic material into cells."
It's been a complex
process to bring the study to MUSC, with more than 400 people having to
be trained in the MUSC family, from the pharmacists and doctors
involved to the person cleaning the OR. Safrit holds up a thick file
folder of all the employees who had to be checked off. Though they
received no extra pay, they were willing to do the training.
"They were enthralled with
what we were accomplishing here," she said. "They were happy to be a
part of it."
The other critical part
that enables the study to proceed are the patients who are willing to
take the risk of a double-blind study, which means they will have their
head shaved and may go through a "sham" surgery where they don't
receive the gene therapy. The patients and researchers won't know until
the end of the two-year study who received the real treatment, although
eligible patients who received the sham surgery will be offered the
therapy at the end if they want it.
Mintzer said it takes a
very strong commitment from the person. It takes a commitment from MUSC
as well.
"A few years ago, this
appeared to be science fiction. I'm excited not only about this
particular study but about the possibilities it opens for us
scientifically. It lays the groundwork for gene therapy for other
organs. There is a path that has been laid out, and that puts MUSC
clearly on the forefront for this type of research and eventually, this
type of therapy."
MUSC has one of the few
neuroscience programs in the country to have neurosurgery working with
neurology and Alzheimer's specialists, which is not a common event, he
said.
"This project requires a
very close collaboration between neurosurgery and the dementia
specialists. We have both together at MUSC. The core of our MUSC
mission is to bring to South Carolina what would not be available if
the resources of a university of this caliber were not here."
For more information about
the clinical trial, call 740-1592, ext. 14 or visit http://academicdepartments.musc.edu/arcp/.
Alzheimer's patients
hope gene therapy study works
Avri Beeri knows his
research.
He can tell you everything
there is to know about Alzheimer's disease—about the myriad of clinical
trials, the vitamins and supplements thought to slow the process, and
how he helped his wife to be part of an elite group of only 50 people
who will be in Phase 2 of the CERE-110 clinical trial. He can tell you
what it's like to live with a spouse who suffers from a disease that
requires him to be watching with "10 eyes all the time what is going
on."
He smiles lovingly at
Dvora, his wife of 44 years, who has returned to MUSC for a check up
following her brain surgery March 29, where she may have received gene
therapy as MUSC's first patient to enroll in the trial. Their hope is
that this therapy will stop and possibly even improve her condition.
 Avri
and Dvora Beeri Avri
and Dvora Beeri
Sporting a new cap to
cover her closely-cropped hair, Dvora smiles at her husband. "I just
need to remember," she said of her decision to enter in a double-blind
clinical study that required her to have a portion of her head shaved
for a treatment she may or may not have received when she underwent
surgery.
Beeri nods at his wife's
response. He needs to know he did everything he could to help her do
just that.
He's the memory keeper for
both of them now. Beeri met his wife when he went to take music lessons
from her brother. "He invited me to come home with him, saying 'I will
teach you some lessons.' I never learned how to play the guitar,
but I learned her," he said, smiling.
Beeri found out his wife
had Alzheimer's disease four years ago, when his life researching the
disease began. He reads everything he can trying to find out what can
break the cycle and stop the disease. "I'm constantly Googling all over
the world to find out what study she could go on."
When he ran across the
nerve growth factor research, he knew he wanted to get her into a
study. On a wait list at Duke University, the couple decided to move
forward with doing the clinical trial at MUSC, where they had
participated in a previous study.
"I was following it very
closely to see where it was going to be, and when I found out it was
going to be here, I thought, 'Good, I know these people.'"
Beeri said they've been to
MUSC so much, his car comes on auto-pilot now. The decision to
participate in the trial was simple for him, but not as much for Dvora.
"She was the one who had to be drilled," he said of the holes that
surgeons have to make for the patient to receive the gene therapy. "I
told her, 'If I were the one who had it, I would do it.' If anything
could stop it, it's worth trying it even with all the pain."
Consulting with their
internist in Charlotte, Beeri said he was relieved it took the doctor
10 seconds to advise to do it. "We talked to him together. He said he
would do it for himself."
Dealing with the disease
takes patience. He advises other caregivers to know how fast the
research changes and how much more there is to know about how to help
those who suffer from the disease. If his wife had the "sham" surgery,
he wants her to get the gene therapy should the trial show promising
results.
Beeri knows it might not
work, but still thinks it was worth doing.
"It will help for the
future, even if it doesn't help us right now."
|



 Dr. Jacobo
Mintzer
Dr. Jacobo
Mintzer Gene therapy involves nerve growth
factor being injected into a critical area of the brain called the
Nucleus Basalis of Meynert.
Gene therapy involves nerve growth
factor being injected into a critical area of the brain called the
Nucleus Basalis of Meynert.
 Avri
and Dvora Beeri
Avri
and Dvora Beeri