|
by Kimberly McGhee
Business Development & Marketing
As patients, we don't
think too much about where our drugs come
from. We just expect them to be available
when we need them.
But what if a new,
potentially life-saving drug for your
condition were not yet available for human
use because its development had become
bogged down because of financial or other
pressures? Even worse, what if a standby
drug, one long used to successfully treat
someone with your condition, were suddenly
not available?
MUSC is working hard on both fronts to
ensure that the drugs you need will be
available. As two articles in the
April/May issue of Progress Notes show,
MUSC is working both to discover and
develop the new drugs of tomorrow and to
ensure an adequate and safe supply of
today's standby medications.
Drug Discovery
 Patents on
drugs last only 20 years and it can take 8
to 12 years to bring a drug to market,
leaving a pharmaceutical company only 8 to
12 years to commercialize the drug. In
that time, it must try to recoup its
initial investment, estimated to be about
$1.3 billion for a new compound. With many
of its "blockbuster" patents expiring, the
pharmaceutical industry has become wary of
investing that much time and money into
the development of a novel compound that
could prove to be a dead end. Instead, it
has begun to collaborate more with
academic medical centers and biotechnology
startup companies to do the initial
legwork. Patents on
drugs last only 20 years and it can take 8
to 12 years to bring a drug to market,
leaving a pharmaceutical company only 8 to
12 years to commercialize the drug. In
that time, it must try to recoup its
initial investment, estimated to be about
$1.3 billion for a new compound. With many
of its "blockbuster" patents expiring, the
pharmaceutical industry has become wary of
investing that much time and money into
the development of a novel compound that
could prove to be a dead end. Instead, it
has begun to collaborate more with
academic medical centers and biotechnology
startup companies to do the initial
legwork.
MUSC is now able to
perform most of the functions typically
performed by a drug company, including
identification of a target (a molecular or
cellular entity important to the pathology
of a disease that can be influenced by a
drug), identification and optimization of
a compound, preclinical studies of the
compound's efficacy and safety and
clinical trials in humans. The further
down the development pipeline that a
researcher can take a promising compound,
the more "de-risked" and therefore
attractive it will be to a pharmaceutical
company. Because commercializing a drug is
prohibitively expensive, the project is
usually passed off to industry before any
phase 3 trials (large multicenter trials)
are undertaken.
Industry benefits from
this collaboration with academia by
assuming a project after some of its risk
has been removed, and academic medical
centers like MUSC benefit by seeing their
research translated more rapidly into
patient care and by recouping their
research investment. And, of course,
patients benefit by having access to
cutting-edge treatments more quickly.
Ensuring Safe, Ample
Supply
In the best of times,
the profit margin for manufacturers of
generic drugs is narrow. Recent changes to
Medicare reimbursement have further
narrowed or erased that margin, leaving
generic manufacturers with little
incentive to continue producing some
drugs.
Injectable drugs, which
can be more difficult and therefore
expensive to manufacture because sterile
conditions must be maintained, have been
the most affected. These include
standard-of-care oncology, anesthetic and
antiviral medications, many of which are
currently in critical shortage nationwide.
Manufacturers either
shut down production of such drugs to
focus on more lucrative medications or
dedicate only one production line to them.
Mergers between pharmaceutical companies
are occurring more frequently, meaning
that duplicate production lines can be
shut down and overall capacity to produce
the drug is decreased. Factories housing
production lines for generics—sometimes
the single production line for a given
generic medication—are sometimes not
properly maintained or updated, meaning
that the shortage of a critical injectable
drug is only a manufacturing hitch or bad
inspection report from the US Food and
Drug Administration away.
MUSC physicians are
collaborating to cope with such drug
shortages. They monitor carefully the drug
needs of patients and the current supply
of such drugs, finding appropriate
alternatives where necessary. They are
also acting nationally to address drug
shortages. Michelle Hudspeth, M.D., chief
of Pediatric Hematology/Oncology at MUSC's
Children's Hospital, testified before
Congress about the consequences of drug
shortages for patient care and some
potential underlying causes, and Heather
Kokko, PharmD, director of Pharmacy
Services at MUSC, helped write an expert
panel recommendation on how to cope with
such shortages.
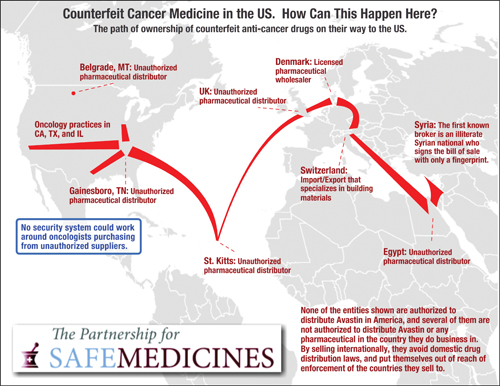
That report warned
against hoarding drugs, which could cause
a patient in need at another hospital to
go without the necessary treatment and
against buying from grey marketers, who
sell drugs of questionable origin and
efficacy. Such marketers capitalize on
shortages to charge exorbitant prices for
drugs that could be stolen, counterfeit or
lacking in efficacy due to improper
storage.
MUSC is working hard
both to design the new, more effective
drugs of tomorrow and to make sure that
the drugs we depend on today are safe and
available when we need them.
To read more about drug
development at MUSC and other stories,
visit the April/May issue of Progress
Notes, available at
http://www.MUSChealth.com/progressnotes.
Editor's
note: "Progress
Notes" is a bimonthly publication
produced by Business Development &
Marketing Services and sent to all
physicians licensed in South Carolina to
inform them about clinical and research
innovations at MUSC.
Friday, June
1, 2012
|



 Patents on
drugs last only 20 years and it can take 8
to 12 years to bring a drug to market,
leaving a pharmaceutical company only 8 to
12 years to commercialize the drug. In
that time, it must try to recoup its
initial investment, estimated to be about
$1.3 billion for a new compound. With many
of its "blockbuster" patents expiring, the
pharmaceutical industry has become wary of
investing that much time and money into
the development of a novel compound that
could prove to be a dead end. Instead, it
has begun to collaborate more with
academic medical centers and biotechnology
startup companies to do the initial
legwork.
Patents on
drugs last only 20 years and it can take 8
to 12 years to bring a drug to market,
leaving a pharmaceutical company only 8 to
12 years to commercialize the drug. In
that time, it must try to recoup its
initial investment, estimated to be about
$1.3 billion for a new compound. With many
of its "blockbuster" patents expiring, the
pharmaceutical industry has become wary of
investing that much time and money into
the development of a novel compound that
could prove to be a dead end. Instead, it
has begun to collaborate more with
academic medical centers and biotechnology
startup companies to do the initial
legwork.